Real-life data in drug development
The Innovative Medicines Initiative (IMI) is a public–private partnership between the EU and the pharmaceutical industry whose mission is to improve health by speeding up the development of, and patient access to, innovative medicines. Launched in 2008, IMI is the largest life sciences partnership of its kind. IMI1 (2008-13) and IMI2 (2014-2020) have a combined budget of over €5 billion, approximately half of which is provided by the pharmaceutical industry. IMI has supported over 140 projects, some focused on specific conditions and others, such as GetReal, on broader challenges in drug development.
The GetReal project, which ran from 2013 to 2017, was a consortium bringing together pharmaceutical manufacturers, academic groups, research agencies, a patients’ organisation (IAPO), small and medium-sized enterprises (SMEs), and regulatory and health technology assessment agencies.
GetReal aimed to show how robust new methods of real-world evidence (RWE) collection and synthesis could be adopted earlier in pharmaceutical research and development (R&D) and the healthcare decision-making process.
The GetReal consortium:
- Brought together healthcare decision makers, academics, pharmaceutical companies, clinicians and other societal stakeholders
- assessed existing processes, methodologies and key research issues
- proposed innovative trial designs and assessed the value of information
- proposed and tested innovative analytical and predictive modelling approaches
- assessed operational challenges, and proposed and tested the impact of solutions
- created new tools to support decision-making, and to use in evaluating development programmes and assessing the value of new medicines
- shared and discussed project outputs with healthcare decision makers, academics, pharmaceutical companies, clinicians and other societal stakeholders
- developed training for researchers, healthcare decision makers and societal stakeholders in the public and private sector to increase knowledge about the use of RWE in demonstrating relative effectiveness of medicines.
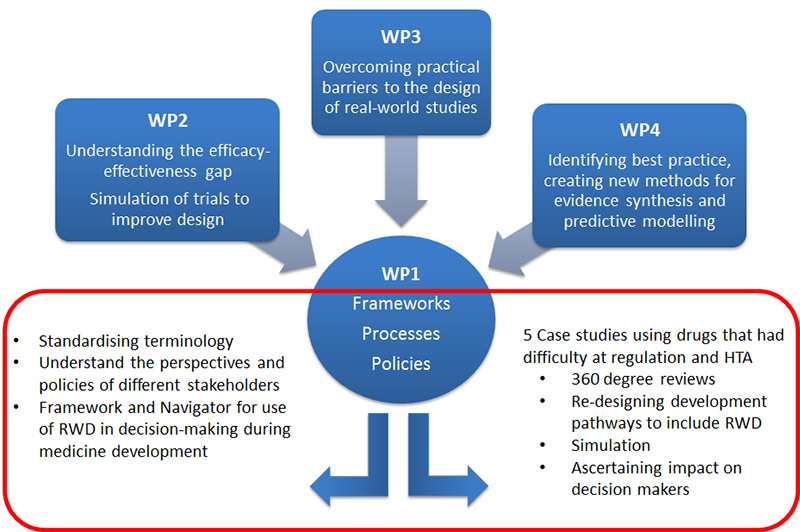
GetReal was organised into four main work packages (see below). Work packages 2-4 (WP2, WP3, WP4) focused on understanding and testing aspects of the use or generation of RWE to support medicine development. Work package 1 (WP1) focused on obtaining the views of stakeholders about the methods and study designs reviewed in GetReal. Decision-making stakeholders are regulatory authorities (marketing authorisation), health technology assessment agencies (reimbursement) and pharmaceutical company R&D (funding of trials and evidence generation). Other stakeholders include patient organisations and clinicians, whose insights inform the decision makers’ perspectives.
The original GetReal project has been followed by the GetReal Initiative, which is running from 2018 to 2020. The objective of this project is to drive the sustainable adoption of tools, methodologies and best practices from GetReal, and thereby increase the quality of real-world evidence generation in drug development and regulatory/health technology assessment processes across Europe.
Image. Organisation of GetReal Work packages